In-Silico Orthopedics

For an efficiency-based individualized therapy, the coherence between product functionality and the patient's pathology must be established. This requires expertise in biomechanics of the human and in structural mechanics of the product, respectively, for an objective analysis processes to enable a well-founded personalization of medical technology that demonstrably enhances the patient's therapy development.
The personalized customization of product functions to the patient necessitates advanced digital personalization technology, achievable only through computer-assisted methods. Due to the resulting complexity of product functions, designing, manufacturing, and digitization, strategic concepts must be developed to pave the way for a digital future. Additionally, the sustainability of product life cycles is becoming increasingly crucial for product acceptance. In this context, bio-based materials are gaining momentum in the market. By incorporating bio-inspired functionality into personalized products, new sustainable solutions can be created. However, for many companies, entering a personalized digital product development strategy is challenging because it requires various cutting-edge technologies from different fields.
Fraunhofer IPA and the »Mass Personalization« competence center are addressing this challenge to reduce existing risks and innovation barriers in the service of Germany's economic development.
Experimental Biomechanics
Experimental analyses of the musculoskeletal system are essential for diagnosing orthopedic pathologies. There are various ways to generate analysis data of the musculoskeletal system, including kinetics and kinematics data from biomechanical motion analysis, medical image data from MRI, CT, X-ray, or ultrasound scans for analysis of the anatomy, pathology or biomechanics of tissues and joints. However, by linking these relevant data, new opportunities arise to uncover pathological connections. For this, all measurement processes must be well-coordinated with each other. Only through precise registration of image and measurement data can further uses of the data be enabled, such as in computer-assisted analysis processes to facilitate informed biomechanical human simulations or for product development beyond diagnostic applications.
Computational Biomechanics
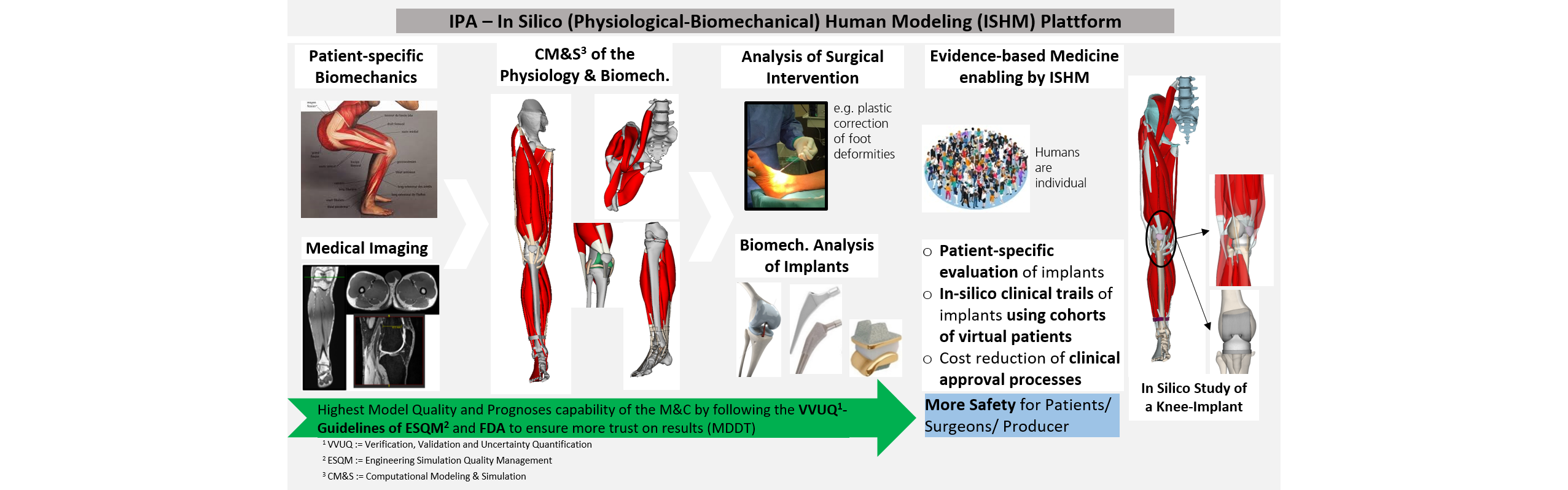
The human musculoskeletal system is a complex system that causes the body limbs to move dynamically through targeted contraction of the muscular system. Muscle contractions result from physiological (electro-chemo-mechanical) processes triggered by neurological stimulation. In many neuromuscular diseases due to paresis, the muscular system is imbalanced and the control of the limbs is disturbed. For the realistic analysis of these pathologies of the muscular system, IPA is developing an in silico physiological-biomechanical human modeling (ISHM) platform, with which the complex musculoskeletal system can be simulated with detailed, prognostic 3D-resolved human models (muscles, tendons, connective tissue, ligaments, skin, etc.). This allows us to study the consequences of interventions in the sensitive muscle system, e.g. by reconstructive surgery such as achilles tendon lengthening, muscle or tendon transfer or ACL replacement. Our goal with the ISHM platform is to introduce more comprehensible evidence into the surgeon's decision-making process.
Personalized medical technology through In-silico human models
For every medical device (implants or orthoses) selected from the product range for a patient (standardised) or manufactured individually (personalized), the question arises as to how great its impact actually is on the development of therapy. What influence does it have on individual biomechanics? Can physiological-biomechanical changes occur that are potentially critical to the musculoskeletal system? Is the personalized product superior to the standard product? In orthopedics, it has been shown that the best therapy is the one that requires minimal support and avoids overcorrection. This is exactly the problem we address with our ISHM platform by testing and optimizing the biomechanical effectiveness of medical devices on a patient-specific manner or for a defined cohort of patients. In addition, the new Medical Device Regulation (MDR) for medical devices requires that medical evidence for each device be provided by clinical studies. However, information on patient-product interactions is often difficult to draw from expensive and complex clinical trials due to the large anatomical and physiological variation among subjects and the limited number of subjects. In silico clinical trials with arbitrarily large virtual patient cohorts can help to make product approval processes more efficient and accelerate them through robust, traceable testing of implants.
Sustainably Bionically-Inspired Products and Production Processes
When it comes to implants, it becomes especially clear that these are not just simple replacement parts that are inserted into the human body like in the case of a car. It has been learned that the body reacts in various and sometimes quite drastic ways to inadequate materials and their properties. The closer the replacement comes to the biological prototype, the more compatible and sustainable it is.
Even with surgical instruments that must perform all the functions used by animals as effectors for grasping, holding, cutting, piercing, drilling, etc., it is worth looking to nature to develop more effective and efficient instruments. Insects provide these effectors in small sizes, as required for endoscopic procedures.
Our scientists take this interdisciplinary approach to nature to develop new and better implants and instruments.